
With 100 mL of HCl (g) to give 100 mL of C 2H 5Cl (g). Calculate pressure volume type of work in it ML of ethylene(g) and 100 mL of HCl (g) are allowed to react at 2 atm pressureĪs per the reaction given below. Indicates the work is done by the system on the surroundings Mole of a gas expands by 3 L against a constant pressure of 3 atmosphere.Ĭalculate the pressure volume work done in a) L-atm b) joules and c) calories. N = 1 mol, V 1 = 20 L, V 2 = 8 L, Work of compression W = +Īns: constant external pressure is 3.74 atm Mole of an ideal gas is compressed isothermally from volume of 20 L to 8 LĪgainst constant external pressure, when pressure volume work obtained is 44.9 The pressure-volume work involved in the process is 36.5 J. N = 3 moles, V 1 = 300 cm 3= 0.3 L, V 2 =Īns: work done = – 4.5 L atm or – 423.4 Jġ mole of an ideal gas is compressed isothermally from a volume of 500 cm 3 against a constant external pressure of 1.216 × 10 5 Pa. To the volume 2.5 L against a constant external pressure of 1.9 atm at 300 K.Ĭalculate the pressure volume work in L atm and J. Moles of an ideal gas are expanded isothermally from volume of 300 cm 3 Indicates the work is done by the surrounding on the system The negative sign indicates the work is done by the system on the surroundingsĢ moles of an ideal gas are compressed isothermally from volume of 10 dm 3 to the volume 2 dm 3 against a constant external pressure of 1.01 × 10 5 Nm -2. Work done in an isothermal process is given by W = – P ext × ΔVĪns: work done = – 4.5 L atm or – 455.8 J Calculate the pressure-volume work in L atm and J. Numerical Problems on Pressure Volume Work:Ģ moles of an ideal gas are expanded isothermally from volume of 15.5 L to the volume 20 L against a constant external pressure of 1 atm. Work of compression is taken as positive (+W).
Thus when the work done on the system by the surrounding i.e. During compression, V 2 Work of expansion is taken as negative (- W). Thus when the work is done by the system on the surrounding i.e. During the expansion, V 2 > V 1, Hence ΔV is positive.Thus work is an organized form of energy. Thus work is a stimulus which increases the organized motion of the molecules of the gas. The movement of the piston makes the gas molecules to move in the direction of applied force. Now let us consider work done on a system by compressing the gas in a cylinder. When heat is added to gas the random motion of molecules of gas increases, thus heat is a stimulus to gas which increases the random motion of the gas molecules. The system from the surrounding (q) is equal to work done (W) by the system on Thus in a cyclic process heat absorbed by
#WORKDONE UNDER GRAPH SERIES#
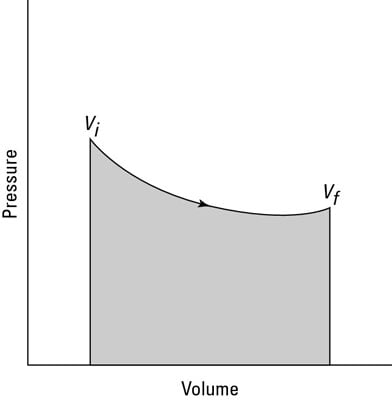
Such anĪ cyclic process is one which consists ofĪ series of intermediate steps, at the end of which the system returns to its Necessary, hence no work is done when a gas expands in a vacuum. When a gas expands in a vacuum there is no opposing Work, therefore, is not a state function.


it is independent of the number of moles) and Temperature of the gas (T).


 0 kommentar(er)
0 kommentar(er)
